What to expect with Centrivive
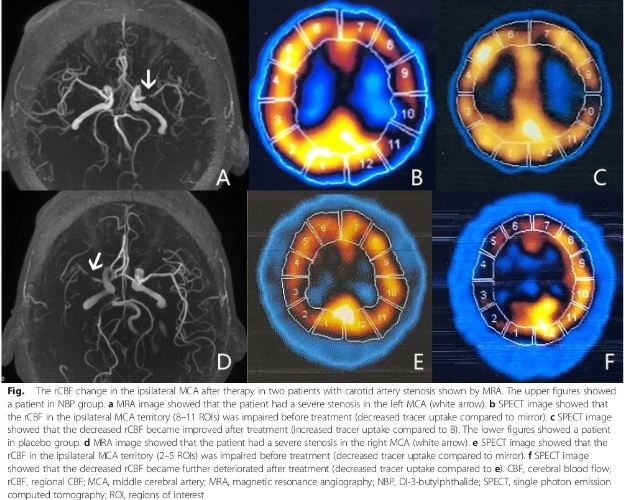
CENTRIVIVE increases perfused vascular density in cerebral infarct area and prevents death and disability in first 90 days of its use following AIS (Acute Ischemic Stroke).
CENTRIVIVE exhibits Multi-Prong actions
1
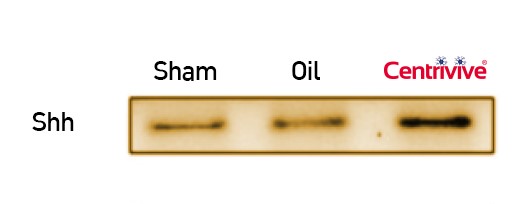
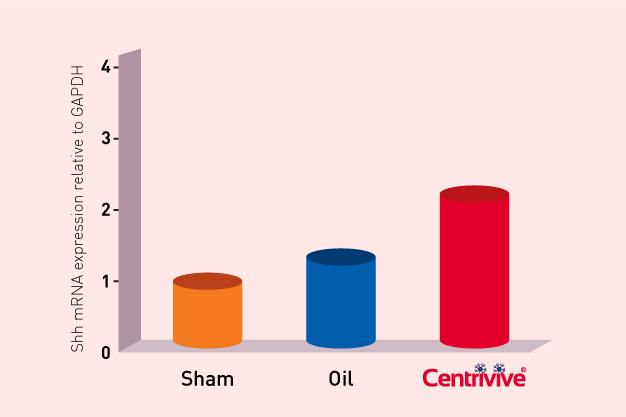
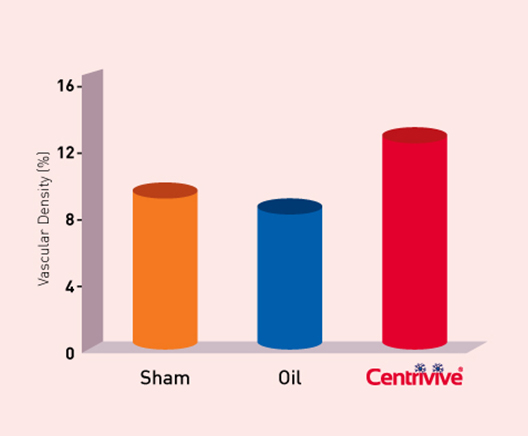
CENTRIVIVE upregulates Sonic Hedgehog (Shh) gene in astrocytes of cerebral infarct by increasing its expression by 200%. Shh is responsible for revival of existing vasculature and forming new collateral vasculature (neovascularisation) to rescue ischemic brain tissue

2
Shh upregulated by CENTRIVIVE causes significant increase in
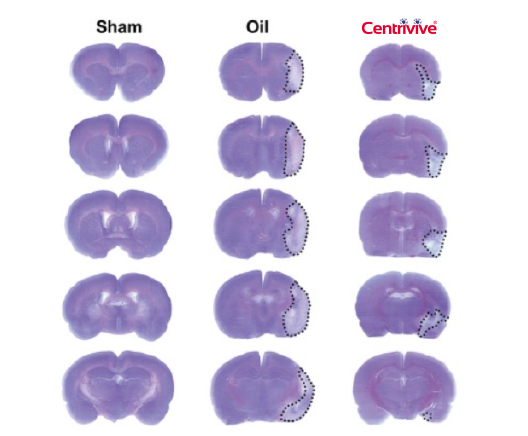
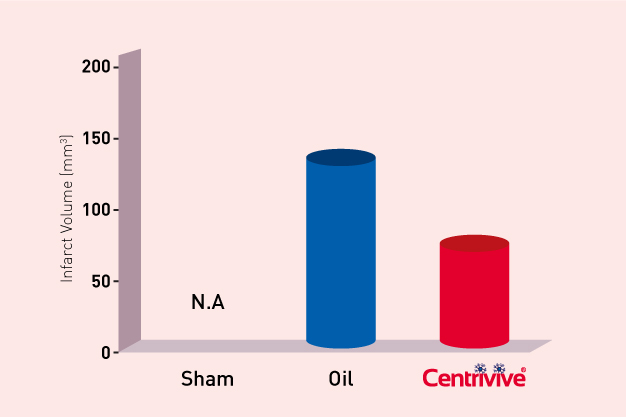
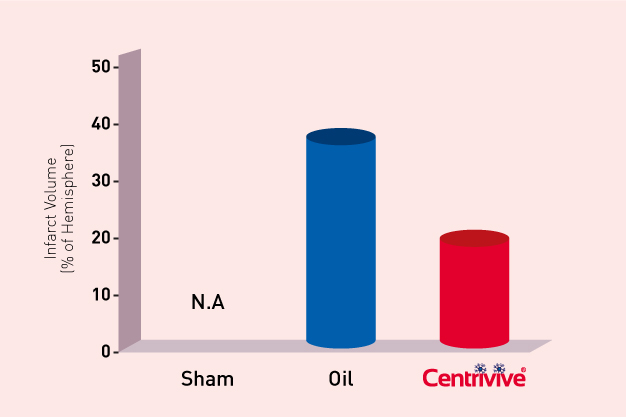
4
Mitochondira Protection
CENTRIVIVE protects mitochondria by activation of Na/K-ATPase and Ca-ATPase & prevents mitochondrial swelling.5
Reduces Brain Edema
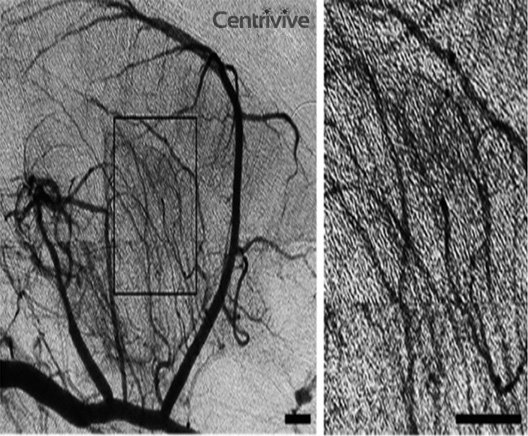
CENTRIVIVE is shown to reduce cerebral edema and protect BBB integrity by inhibiting MMP-9 & down-regulation of Claudin-5 . It also up-regulating TIMP-1 which is a specific inhibitor of MMP-9.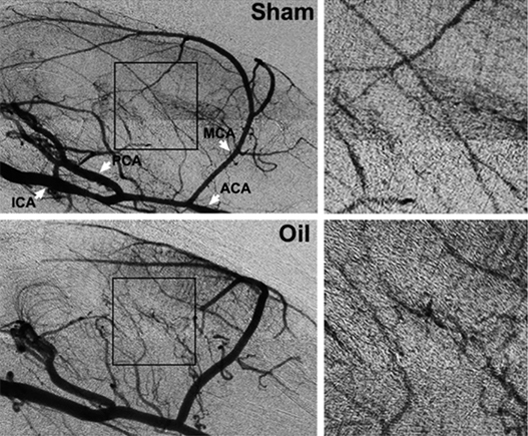

Other Conditions CENTRIVIVE Treats
Apart from Acute Ischemic Stroke (AIS)- CENTRIVIVE was found to be efficacious and safe in other neurological conditions mentioned below
About Vascular Dementia
Vascular cognitive dysfunction refers to memory loss due to vascular factors, and cognitive dysfunction and an altered mental status are common clinical manifestations. The disease may gradually worsen without intervention, leading to severe economic and medical burdens on society and families. Vascular cognitive dysfunction is second only to Alzheimer’s disease as a leading cause of senile dementia.
CENTRIVIVE is known to increase vascular density alongside strong anti-oxidant and mitochondria protection action. A total of 60 patients with vascular cognitive dysfunction were randomly divided into a CENTRIVIVE group and a control group, with 30 patients in each group. Patients in both groups were given conventional basic treatment which included blood pressure control, blood sugar control, and lipid-lowering therapies, as well as treatment for myocardial ischemia. The treatment group was also given CENTRIVIVE soft capsules 200 mg, 3 times daily for 14 days. CENTRIVIVE treatment achieves higher scores of HDS, MMSE and ADL scores which provided good evidence of the effectiveness of CENTRIVIVE therapy in the management of vascular cognitive impairment. Results were significant at day 14.
About Parkinson’s Disease
CENTRIVIVE alleviates oxidative damage and mitochondrial dysfunction. It has been revealed to reduce the loss of dopamine neurons in pre-clinical PD models. A randomized, controlled trial was performed between September 2014 and December 2016. Pairs of patients matched by age, gender and off medication Unified PD Rating Scale motor subscale (UPDRS-III) scores, were randomly assigned to CENTRIVIVE treatment group and a control group. All patients continued their originally prescribed medication regimen and those in the CENTRIVE group were administered CENTRIVIVE at 200 mg three times daily for 24 weeks. Primary outcome measures were changes in UPDRS-III, including tremor score and non-tremor score, the Pittsburgh sleep quality index (PSQI) and the PD 39-items questionnaire (PDQ) scores.
Assessments were completed by blinded evaluators at baseline and 12, 24 and 48 weeks after randomization. All patients were monitored for adverse events (AEs). A total of 103 patients were enrolled in the present study. The CENTRIVIVE group exhibited significantly greater improvements in the non-tremor, PSQI and PDQ-39 scores than the control group, which generally exhibited no improvement. Centrivive-associated AEs were uncommon and primarily consisted of mild gastrointestinal symptoms. In conclusion, over the 6-month treatment period, CENTRIVIVE was safe and effective for improving the symptoms and impairing the progression of patients with PD.
